Student team Better/e is working on alternative to lithium-ion batteries

Student team Better/e is working on a battery of which iron is the main component. They see this as the future of large-scale energy storage.
With the energy transition, we are at the dawn of an era in which lithium has a major role to play. It is the lightest of all metals and main component of numerous batteries. But if we fail to act, we will already have a shortage of it by 2050, scientists warn. According to them, the solution lies in improving the recycling of batteries and the development of new types of batteries based on other raw materials.
That last option is exactly what the Better/e student team from the Eindhoven University of Technology (TU/e) is working on.
“There is not enough lithium on earth. We want to develop a technology whereby the basic raw materials are available in large quantities, which are also inexpensive and can be used on a large scale.”
Goof Roelands, student chemical engineering TU/e
He is a chemical engineering student at TU/e and responsible for the chemical side of the story at Better/e.
Flow battery
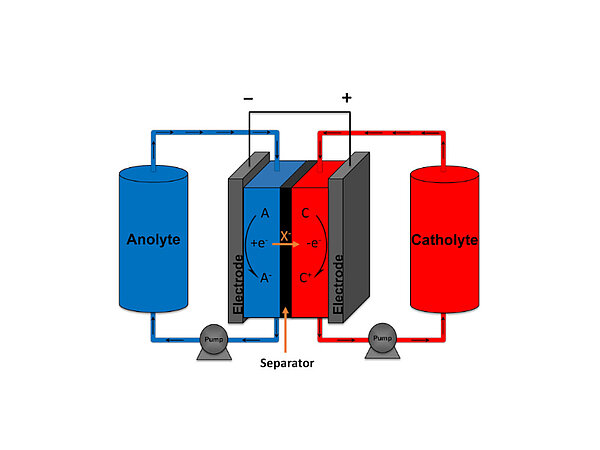
The team is developing an iron redox flow battery that stores energy in electrochemical form. A flow battery is made up of two tanks that contain two highly conductive electrolyte liquids separated by an electrochemical cell. The system generates electrical current this way by exchanging negatively and positively charged fluids. That current is captured by electrodes and can then be stored and subsequently used in electrical systems. The two liquids are separated by a membrane.
The main disadvantage of these type of flow batteries is that they use expensive fluids. The flow battery that is most commonly used now works on the basis of vanadium, a medium-hard and scarce sort of metal. Better/e aims to develop a flow battery whose main component is iron. Much larger amounts of this are available and are consequently much cheaper.
Roelands: “We are now working on building the prototype of a single cell. That should be ready by the end of this year. You can add certain substances, such as acid, to the cell so that the reaction process runs more efficiently. We are still weighing up which acid we should use. After all, the substance shouldn’t be too expensive either and it has to be available in large quantities.”
Since Roelands joined the team a year ago, the work mainly involved doing research into sources. Not the most exciting part of the process, but as a result, the team now has a solid foundation on which to develop a cell that is efficient enough. At the same time, this is also the greatest challenge. This is because as soon as the pH value of the liquids is too low, a hydrogen evolution reaction takes place. This causes the efficiency of the cell to deteriorate. An acidity higher than three is also not desirable. “Then a precipitation reaction takes place, causing all the substances in the solution to sink to the bottom and form a layer of solids. The main technological challenge is to find a good balance between these things.”
Large-scale application
Once the single cell is ready, Better/e wants to put multiple cells together in a stack design so that more electricity can be stored in the system. The technology is not intended for small-scale applications, such as phones or cars. “The membrane that separates the two compartments is always going to be relatively large relative to the compartments on such a small scale. Then the chemical reaction would be inefficient. It is only when you have large containers with multiple cells that the technology becomes interesting. Then you can put pumps in there and control everything in a very efficient way. All the electricity that solar panels and wind turbines bring in can then be converted and stored with our technology so that it can then be used in the city."
"Our battery is the future of energy storage on a large scale."
Goof Roelands, responsible for the chemical side at Better/e
Not profit, but an open source document as the end goal
The ultimate goal is to publish an open source document that everyone can access. “By doing that, we hope that the research on this technology will gain momentum. We are keen to make the technology accessible to a large target group as soon as possible. So our goal is not to grow into a company ourselves, we are really doing it for the greater good.” Better/e is working together with Fair Battery, another team from TU/e who are also working on developing a redox battery.
Last summer, Better/e won the Boost Your Tech Career award at the TU/e Contest and placed second in the overall prototype category. “We gained a lot of new contacts, which is valuable in any event. And as a result of winning the BYTC award, we are allowed to follow free courses to learn how to weld, for instance. That’s also something we can really use. I’m a chemical engineer myself, so it’s nice that through these courses we will soon be able to help mechanical engineers better.”