Smart monitor saves babies’ lives in Africa
More than thirty babies on an incubator ward with a single nurse. This is quite normal in African hospitals. The nurse is always busy with the day-to-day care. Identifying early symptoms of disease is difficult then. Monitors for keeping an eye on the heartbeat or blood pressure (such as we are used to in the western world) are often too expensive and complex. That’s why so many babies die needlessly from treatable conditions such as blood poisoning, dehydration and pneumonia.
Start-up GOAL 3 wants to do something about this. The company is named after the third sustainability goal of the United Nations: Healthcare for everyone. The focus is primarily on Africa because improving healthcare there is extremely important at the moment. As a tropical doctor, Co-founder Niek Versteegde has seen this with his own eyes in an incubator ward in Tanzania.
What are you doing to resolve this issue?
“Three years ago, Bart Bierling, one of the co-founders of GOAL 3, started a project which involved the monitoring of patients in Africa,” Versteegde explains. “I joined up with that. We can detect problems earlier and treat them at an earlier stage by monitoring patients more effectively.”
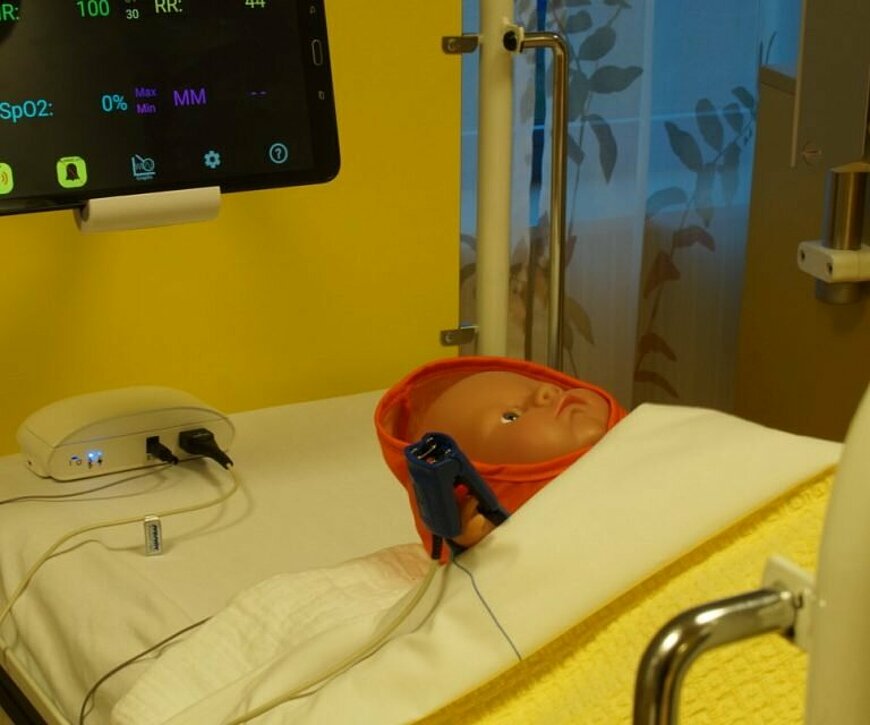
“We first looked at a monitor that is capable of measuring vital parameters such as heart rate and breathing. The monitor must be robust and affordable for use in developing countries. Additionally, the monitor transmits data readings to a tablet. An algorithm analyzes the data to help doctors there interpret the data. For instance, the algorithm is able to recognize signs of a specific disease. This way, serious infections such as blood poisoning can be identified before they become a real problem. This allows the physician to take follow-up action much faster and lives are saved as a result.”
“That kind of algorithm doesn’t exist yet. Monitors only sound an alarm, e.g. if the heart rate is above a certain value. This isn’t enough for developing countries. The doctor needs more support, because there is either insufficient expertise available or because the workload is too high. This technology can provide that support.”
What is your ultimate goal with this monitor?
“We want to improve the quality of care in developing countries with this monitor. This enables us to prevent children from dying unnecessarily from diseases that can be treated properly. We are currently focusing on recognizing and treating blood poisoning. But in the future we also want to look at conditions such as pneumonia and dehydration. The monitor can then measure oxygen levels in the blood, heart rate, breathing and movement, for example. The algorithm is subsequently able to recognize patterns in these values. This is how it can support doctors in making decisions.”
What was the main obstacle that you had to overcome?
“It’s hard for us to really relate the message around the technology so that, for example, partners can be brought on board. We need to keep on improving and tightening up this message. Any steps that the company takes during the development process must be in line with this and be made in the right order. This side of business development is still quite tricky at times because we are all more concerned with technology and healthcare.”
Besides the more difficult times, there’s also occasions for breaking out the champagne. When did you do that?
“When we were told that the grant had been approved for a clinical trial in Malawi in conjunction with the Academic Medical Center. We’re launching this in April of this year.”
What can we expect from you in the coming year?
“We’re going to carry out the study in Malawi first. After that, we want to make another prototype so that we can test the product on a larger scale before we launch it on the market.”
“We want to bring the product to the market in the coming years. Initially, we will mainly focus on our robust monitor. We want to add the algorithm to the system later on. Hardware is easier to certify because there are already similar products out there. The algorithm needs to be tested much more extensively first before we will be allowed to use it. We want the device to comply with Dutch standards. The expectation is that the algorithm will be ready for use around 2023.”


Article by Innovation Origins.